Zydus Life receives final USFDA approval to market acne-treating gel
Summary
Zydus Life Share Price | The gel is used to treat acne and will be manufactured at the pharma company’s topical manufacturing facility at Changodar in Ahmedabad.
Zydus Life on Thursday, May 9, said it has received the final approval from the US Food and Drug Administration (USFDA) to market the Dapsone Gel, 7.5%.
The gel is used to treat acne and will be manufactured at the pharma company’s topical manufacturing facility at Changodar in Ahmedabad.
As per the IQVIA MAT March 24, the Dapsone Gel had 7.5% annual sales of $35.8 million in the US.

The company now has a total of 395 approvals and has filed more than 460 ANDAs since the beginning of the filing process in FY2003-04.
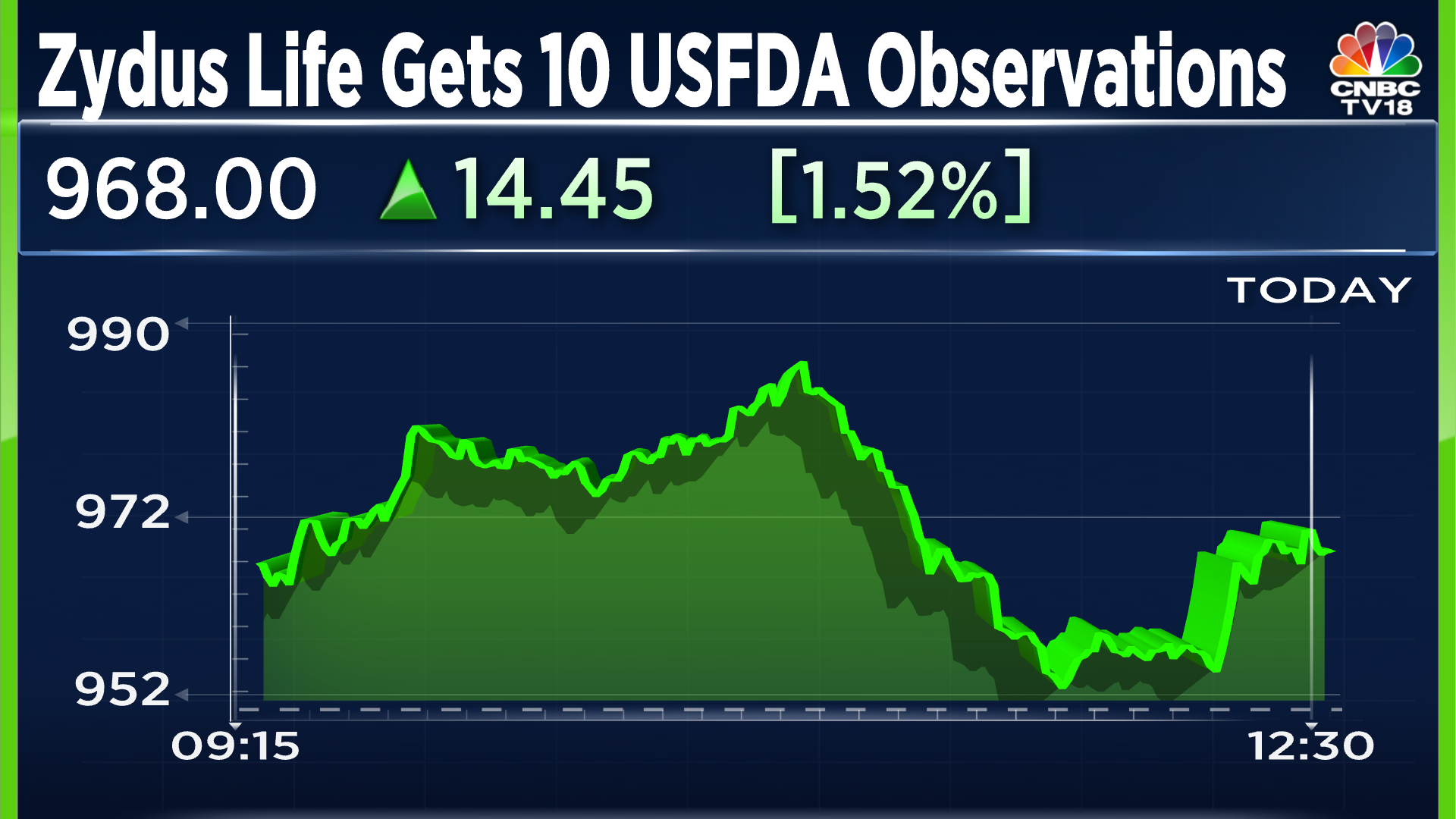
On another note, last week, CNBC-TV18 accessed the 10 observations the USFDA had issued for Zydus Life’s Jarod facility. The USFDA had inspected the facility from April 15 to 23. The observations stated that the facility failed to establish procedures to establish purity and quality of products, employees engagid in manufacturing drug product lacked the required training, the sampling plans and test procedures were not followed, among others.
The company had said it will work closely with the USFDA to address and respond to the observations.
Last month, the company also launched a tablet — Mirabegron Extended-Release tablets, 25 mg — in the US market to treat overactive bladder. It is to be manufactured at the pharma’s company’s manufacturing facility in Ahmedabad SEZ.
Zydus Life shares were trading 0.43% lower at ₹1001.3 apiece at 11.55 am. The stcok has gained 63.6% in the past six months and 90.9% in the past year.
Also Read: SKF India gains over 11% on 43% surge in Q4 net profit

Elon Musk forms several ‘X Holdings’ companies to fund potential Twitter buyout
3 Mins Read
Thursday’s filing dispelled some doubts, though Musk still has work to do. He and his advisers will spend the coming days vetting potential investors for the equity portion of his offer, according to people familiar with the matter









 Listen to the Article
Listen to the Article  Daily Newsletter
Daily Newsletter