Pfizer declares interim dividend of Rs 30 per share after sale of Upjohn business
Summary
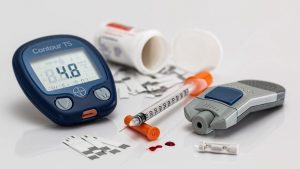
Shares of Pfizer Ltd ended at Rs 4,306.75, up by Rs 28.15, or 0.66 percent on the BSE.
The US-based pharma major Pfizer Ltd on Tuesday said it has declared an interim special dividend of Rs 30 per equity share for the financial year ending March 31, 2023.
In a regulatory filing, the company said, “The Board of Directors at its meeting held today, i.e., September 6, 2022, has declared an Interim (Special) Dividend of Rs. 30/- per equity share of Rs. 10/- each (300%) for the financial year ending March 31, 2023, in view of the gain on account of sale of Upjohn Business to Mylan Pharmaceuticals Private Ltd.”
This is the first interim dividend the company has announced for this fiscal. September 20, 2022, has been the record date for the purpose of payment of dividends.
Also Read: Reliance Industries expands new energy presence with SenseHawk deal
Back in 2021, Pfizer had entered into an agreement with Mylan Pharmaceuticals to sell its Upjohn business for Rs 180.48 crore.
“The Upjohn business comprises six brands which include Lyrica, Viagra, Celebrex, Amlogard, Daxid and Dilantin. These along with related business assets and liabilities will transition to Mylan,” the filing said.
The rationale for the transaction is that Pfizer Inc, USA, on July 29, 2019, announced that it had entered into a definitive agreement to combine its Upjohn business — which consists of off-patented branded and generic established medicines — with Mylan N.V., thereby creating a new global pharmaceutical company, Viatris Inc.
Also Read: A Kolkata-based lender’s valuation is catching attention on Dalal Street — here’s why
Under the global transaction, six brands currently marketed by Pfizer Ltd in India, viz., Lyrica, Viagra, Celebrex, Amlogard, Daxid and Dilantin, will transition to Mylan Pharmaceuticals.

Elon Musk forms several ‘X Holdings’ companies to fund potential Twitter buyout
3 Mins Read
Thursday’s filing dispelled some doubts, though Musk still has work to do. He and his advisers will spend the coming days vetting potential investors for the equity portion of his offer, according to people familiar with the matter








 Listen to the Article
Listen to the Article  Daily Newsletter
Daily Newsletter