COVID vaccine: AIIMS-Delhi begins recruiting volunteers for Covaxin human trials

KV Prasad Jun 13, 2022, 06:35 AM IST (Published)
 Listen to the Article (6 Minutes)
Listen to the Article (6 Minutes)
Summary
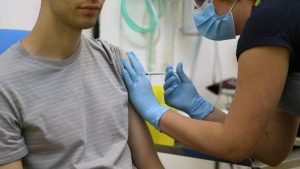
AIIMS-Delhi on Monday began the process of recruiting volunteers for conducting human clinical trials of the indigenously developed COVID-19 vaccine candidate Covaxin, the premier medical institute’s director Dr Randeep Guleria said.
AIIMS-Delhi on Monday began the process of recruiting volunteers for conducting human clinical trials of the indigenously developed COVID-19 vaccine candidate Covaxin, the premier medical institute’s director Dr Randeep Guleria said.
He also said that there is not much evidence of community transmission of COVID-19 happening at the national level.
AIIMS-Delhi is among the 12 sites selected by the Indian Council for Medical Research (ICMR) for conducting Phase I and II randomised, double-blind and placebo-controlled clinical trials of Covaxin.
In phase I, the vaccine would be tested on 375 volunteers and 100 of them, the highest, would be from AIIMS. The second phase, would include around 750 volunteers from all 12 sites put together, Guleria said while addressing reporters.
Phase I of the vaccine trial will be done on healthy people, aged between 18 and 55 years, having no co-morbid conditions. Women with no pregnancy will also be selected to be a part of the trial in the first phase, he said.
In the second phase, 750 people will be recruited and they will be between 12 and 65 years of age, Guleria said.
Already, around 1,800 people have registered for the trial at AIIMS, he said.
“In the first phase, we see the safety of the vaccine which is of primary importance and the dose range is also calculated,” the AIIMS-Delhi director said.
In the second phase, we see how immunogenic it is and that is subsequently followed by phase III, which involves a larger population to assess its efficacy and therapeutic benefit”, Guleria said.
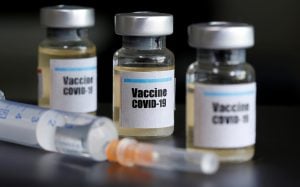
Covaxin, which is being developed by Hyderabad-based Bharat Biotech in collaboration with the ICMR and the National Institute of Virology (NIV), had recently got the nod for human clinical trials from the Drugs Controller General of India (DCGI).
To a question, he said there is not much evidence of community transmission of COVID-19 happening at the national level. “But there are hotspots, even in cities where there is a spike in cases and it is likely that local transmission is happening in those areas,” Guleria said.
Asked when a vaccine would be available, he said it will all depend on whether everything works out in a good manner.
It is possible that “we may say that the vaccine is safe and then we find that it is not giving that much efficacy. (Then) we do something more that may take a few more months,” he said.
“So, predicting exactly when the vaccine is going to be ready is a difficult business. But then say if everything works out ideally, then by the end of the year or early next year, we should be in a position to say that we can start manufacturing. But then there are lot of ifs and buts in that,” Guleria said.
Asked if COVID-19 cases in India have hit the peak, he said, “My feeling is that certain areas have hit their peak. Delhi seems to have done so because the cases have declined significantly. But certain areas are yet to reach the peak .
Cases are increasing in certain states and they will reach a peak a little later, Guleria said, adding that some states in the south, some places in central Mumbai, and Ahmedabad seem to be showing a decline in cases.
“But then having hit the peak and then come down does not mean that you have to let your guard down. In many cities outside India, especially in the US, when people felt the peak was over and started not following social distancing and not wearing masks, there was a spike in cases,” he said.
Guleria underlined that even if cases come down, infection control and containment measures along with other dos and don’ts have to be followed.
Phase human trials have started. This is something which is very heartening because this is an indigenous vaccine. In India we don’t do research and development, he said
Making a new vaccine is a great achievement for India and we are now into research and development, making our own vaccine and being able to then mass produce it , Guleria said.
We are very good in mass production. Even if a vaccine comes from any part of the world, India will be involved in its production because 60 per cent or more of the vaccines of the world are being made in India, the AIIMS-Delhi director said.
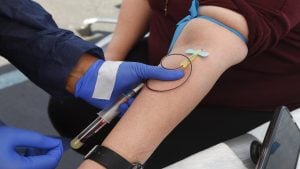
Professor at the Centre for Community Medicine at AIIMS Dr Sanjay Rai said on Monday, AIIMS-Delhi started the registration and screening of individuals, and evaluating their health condition before vaccinating them.
There are three formulations of the vaccine, and each subject will be given any one of the formulations in two doses in two weeks apart. The first 50 will get the lowest strength dose of the vaccine. If it is found to be safe in them, then it will be given to another 50 patients in high doses, said Rai, who is the principal investigator of the trials.
The minimum time required for Phase I trial results to come out will be four to six weeks, he said.
Anybody willing to participate in the trial can send an email to Ctaiims.covid19@gmail.com, or an SMS to or call to 7428847499, Rai said.

Elon Musk forms several ‘X Holdings’ companies to fund potential Twitter buyout
3 Mins Read
Thursday’s filing dispelled some doubts, though Musk still has work to do. He and his advisers will spend the coming days vetting potential investors for the equity portion of his offer, according to people familiar with the matter

KV Prasad Journo follow politics, process in Parliament and US Congress. Former Congressional APSA-Fulbright Fellow









 Listen to the Article
Listen to the Article  Daily Newsletter
Daily Newsletter