One year of vaccination: Great feat, scope for further improvement

KV Prasad Jun 13, 2022, 06:35 AM IST (Published)
 Listen to the Article (6 Minutes)
Listen to the Article (6 Minutes)
Summary
Amidst a surge in the number of cases, India has started giving booster doses of the COVID-19 vaccine to priority groups from January 10. Health and frontline workers besides people over 60 years of age with comorbidities are eligible for the booster.
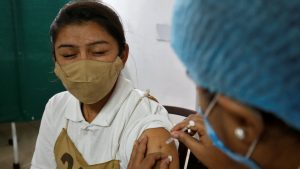
India completed one year of its vaccination drive last week even as the country continues to be in the grip of a third wave of pandemic. When the pandemic had hit the country, very little was known about the virus and the vaccine. But in a very short span of time, the scientists could carve a trajectory for indigenous vaccine.
India began its vaccine campaign on January 16, 2021 and the first doses were administered to its healthcare workers, fighting the battle from the front. The drive was later expanded to cover frontline workers, followed by people above 60 years of age and those above 45 years with comorbidities. Later, the vaccine coverage was also expanded to all above 45 years of age and recently to those above 18 years of age. The country started vaccinating its teenagers between the age group 15-18 on January 3, 2022.
As on January 18, more than 158 crore vaccine doses have been administered so far under the Nationwide Vaccination Drive. Around 907 million persons 15 and older have received at least one shot, 656 million two doses, while 4. 3 million a third dose. Considering the challenges — population size, remote locations and patchy healthcare infrastructure — India’s achievement in such a short time is commendable.
Amidst a surge in the number of cases, India has started giving booster doses of the COVID-19 vaccine to priority groups from January 10. Health and frontline workers besides people over 60 years of age with comorbidities are eligible for the booster.
The booster dose that has been termed as the ‘precaution dose’ will be the same vaccine that was given for the first and the second doses. As on January 18, over 50 lakh healthcare and frontline workers besides citizens aged 60 years or above have received the precaution dose.
“I salute each and every individual who is associated with the vaccination drive. Our vaccination programme has added great strength to the fight against COVID-19. It has led to saving lives and thus protecting livelihoods,” Prime Minister Narendra Modi had tweeted on the completion of one year of the vaccination drive on January 15.
India is likely to begin inoculating children in the 12-14 age groups against Covid from March. Of the estimated 7.4 crore children in the 15-18 age bracket, over 3.45 crore have already received the first dose of Covaxin.
ALSO READ | COVID-19 health insurance: Options available, list of policies and other questions answered
India has witnessed a flurry of research activities on developing the vaccine. The country has been administering two locally made vaccines — Covaxin and Covishield since the vaccination drive kicked off in January 2021. In December, India, approved two new vaccines — Serum Institute of India’s Covovax and Biological E’s Corbevax, both authorised for restricted use in an emergency situation. There are also other candidates which are in different stages of trials in India to test safety and efficacy.
The omicron variant was first discovered in South Africa in November last year. As on January 18 around 70.54 crore total tests had been conducted so far, 16,49,143 tests conducted in the last 24 hours. The daily positivity rate stands at 14.43 percent. India’s active caseload currently stands at 17,36,628. The active cases stand at 4.62 percent. More than 8,890 Omicron cases had been detected so far.
Challenging terrain
The vaccination drive has been hamstrung with several hiccups and challenges. The drive has been dotted with challenges—population, remote terrains and anaemic infrastructure.
While India had been at the forefront of child immunisation, adult immunisation of such a grand scale was an unexplored territory. Besides the logistics pinpricks, establishing a harmonious public-private partnership was a daunting task. The success of a vaccination program of this scale also required immense collaboration between the central and state governments. An effective communication strategy and a robust awareness generation programme also helped in giving the momentum to the vaccination drive.
ALSO READ | EPFO withdrawal: How to avail COVID-19 advance twice from PF account?
The vaccines require an excellent cold chain management and the transportation of the vaccines across the length and breadth of the country was mired with hurdles. The immunisation drive also required an excellent vaccine management strategy.
Way forward
Despite the robust numbers, there is still a lot of scope to build up the data regarding vaccination. This can help to develop the future vaccines for new and other emerging variants. The Indian vaccine developers should work together to build an ecosystem that can fight the future variants.
The concept of mix and match of vaccines also needs to explored broadly to give a further fillip to the drive. Communication programmes for accelerating the vaccination should also be spruced.
—The author, Dr Vanita Srivastava, is an independent science and health writer. Views expressed are personal
Click here to read the author’s other articles

Elon Musk forms several ‘X Holdings’ companies to fund potential Twitter buyout
3 Mins Read
Thursday’s filing dispelled some doubts, though Musk still has work to do. He and his advisers will spend the coming days vetting potential investors for the equity portion of his offer, according to people familiar with the matter

KV Prasad Journo follow politics, process in Parliament and US Congress. Former Congressional APSA-Fulbright Fellow










 Listen to the Article
Listen to the Article  Daily Newsletter
Daily Newsletter