Aurobindo Pharma now weighs mega IPO of Eugia Pharma Specialities to raise around ₹4,500 crore

KV Prasad Jun 13, 2022, 06:35 AM IST (Published)
 Listen to the Article (6 Minutes)
Listen to the Article (6 Minutes)
Summary
“With the markets in a buoyant phase and pharma valuations looking attractive, Aurobindo Pharma is now evaluating a listing of Eugia. The deal was kicked off recently with all the advisors,” said one of the persons.
Aurobindo Pharma is in value unlocking mode again, and is now exploring a big-bang initial public offer (IPO) of its wholly-owned subsidiary — speciality generic firm Eugia Pharma Specialities — to raise around ₹4,500 crore in 2024, multiple industry sources told Moneycontrol.
The Hyderabad-based parent had previously attempted the sale of Eugia and held discussions with top private equity players in 2022, but the negotiations did not fructify into an eventual deal due to differences over valuations.
“With the markets in a buoyant phase and pharma valuations looking attractive, Aurobindo Pharma is now evaluating a listing of Eugia. The deal was kicked off recently with all the advisors,” said one of the persons above.
A second person told Moneycontrol that the plan is to raise around ₹4,500 crore via the proposed IPO. He added that investment banks Axis Capital, Jefferies, Kotak Mahindra Capital and JP Morgan had been shortlisted as advisors along with three law firms. Three other persons also confirmed the above.
Also Read: Bajaj Auto share buyback: Why the company is offering top dollar for your stock
“The plan has shifted to the public markets where there is an ongoing wave of new issues. These are early days and the aim is to raise between ₹4,000 crore to ₹5,000 crore via the listing,” one of them said, warning that the quantum had not been finalized and may change later depending on market conditions
All the five persons cited above spoke to Moneycontrol on the condition of anonymity. Moneycontrol has sent reminders and is awaiting the response to a detailed email query sent to Aurobindo Pharma.
This article will be updated as soon as we hear from the pharma major. An immediate response could not be elicited from Axis Capital, Kotak Mahindra Capital, Jefferies and JP Morgan.
The domestic pharma and life sciences sector has been active in terms of public issues in the last 8 months. Last month, Bain Capital-backed Emcure Pharma re-filed papers with Sebi for an initial public offer with a reduced size.
Mankind Pharma galvanized the domestic IPO market last year with a blockbuster debut in May. It was followed by healthy debuts in the segment from Concorde Biotech, Blue Jet Healthcare and Innova Captab.
Eugia rejig: The Aurobindo Pharma strategy
In a disclosure to the bourses on August 25, Aurobindo Pharma said, “The company has already informed the stock exchanges on August 12, 2023, as part of the outcome of its board meeting that it is in the process of exploring all the possibilities of restructuring the business of Eugia Pharma Specialities Limited, a wholly owned subsidiary company focused on sterile/injectables, oncology and hormonal products.”
Also Read: Reliance Group market capitalisation crosses ₹20 lakh crore; RIL at record high
“And as stated earlier, the Committee of Independent Directors will pursue a comprehensive evaluation of various alternatives or options and recommend to the Board for engendering the next horizon of growth as also enhancing value creation to the stakeholders,” it added.
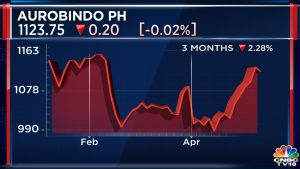
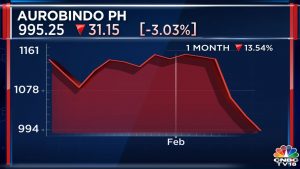
The statement was a clarification of a report by The Economic Times, which said Eugia Pharma had been put on the block once again. Incidentally, Aurobindo Pharma’s stock price has risen by more than 50% in the last six months.
In May 2021, in a move to improve operational efficiency, the pharma firm approved the transfer of its injectable assets into Eugia Pharma Specialities Limited for “greater focus, attention and specialization” and also to “augment fundraise and strategic tie-ups in future through joint ventures etc” according to exchange disclosures.
According to Aurobindo Pharma’s latest annual report, Eugia Pharma, a speciality generic pharmaceutical company, has six manufacturing units located in different locations in India and the US.
Also Read: Vibrant Gujarat Summit 2024: Adani to invest Rs 2 lakh crore in state in 5 years, create 1 lakh jobs
The units contribute to the production of a diverse range of differentiated and unique products such as general injectables, oncology, hormonal, penems, penicillin and ophthalmics. According to the Eugia website, the firm is present in 120 plus countries and has 4,000 plus employees and 250 plus globally approved generics.
Eugia’s parent parent Aurobindo Pharma was founded in 1986 by P.V. Ramprasad Reddy, K. Nityananda Reddy and a small group of professionals.
According to the firm’s website, in addition to being a leading player in semi-synthetic penicillin, Aurobindo Pharma has a presence in key therapeutic segments such as neurosciences (CNS), cardiovascular (CVS), anti-retroviral, anti-diabetics, gastroenterology and anti-biotics.

Elon Musk forms several ‘X Holdings’ companies to fund potential Twitter buyout
3 Mins Read
Thursday’s filing dispelled some doubts, though Musk still has work to do. He and his advisers will spend the coming days vetting potential investors for the equity portion of his offer, according to people familiar with the matter

KV Prasad Journo follow politics, process in Parliament and US Congress. Former Congressional APSA-Fulbright Fellow










 Listen to the Article
Listen to the Article  Daily Newsletter
Daily Newsletter