Who can’t receive COVID-19 nasal vaccine — From benefits to side effects, here’s your one-stop guide

KV Prasad Jun 13, 2022, 06:35 AM IST (Published)
 Listen to the Article (6 Minutes)
Listen to the Article (6 Minutes)
Summary
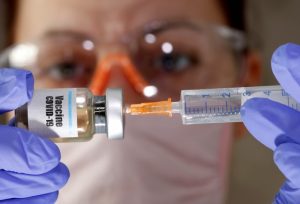
COVID-19 in India: The government has approved Bharat Biotech’s COVID-19 nasal vaccine — BBV154 or iNCOVACC — as a “heterologous booster dose” and it is recommended to be taken as the “first booster dose” only, according to reports.
India’s first COVID-19 nasal vaccine — BBV154 or iNCOVACC — will soon be available in the country and will be used as a booster dose. It will be available at government, as well as, private hospitals and people can book the precautionary dose at CoWin. Bharat Biotech’s nasal vaccine could prove to be a big boost to India’s vaccination programme as it eliminates needle-associated risks and could even “cut into vaccine hesitancy“.
What are the other benefits of this nasal vaccine? When will it be available in the market? How much will it cost? And who can use it? Here’s your one-stop guide:
What is Bharat Biotech’s BBV154 or iNCOVACC?’
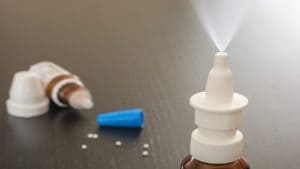
iNCOVACC is an intranasal vaccine for “active immunization” against SARS-CoV-2 virus infection.
How many doses of iNCOVACC can be taken and how is it administered?
iNCOVACC® is administered through the nose, as a two-dose series, four weeks apart. The vaccination course iNCOVACC consists of two separate doses of 0.5 mL of eight drops with four drops indicated for each nostril.
Recently, the government has approved it as a “heterologous booster dose” and it is recommended to be taken as the “first booster dose” only.
Who can receive the naval vaccine?
As of now, the intranasal vaccine is recommended as one of the options for precautionary doses for those aged 18 years and above in India. Those who have been vaccinated with two doses of either Covishield or Covaxin can opt for iNCOVACC as a precautionary dose.
But, if a person has already taken a precautionary or booster dose, iNCOVACC is not recommended for them as the next vaccine shot. In an exclusive interview with NDTV, Dr NK Arora, the head of the country’s vaccine task force, said, “It (nasal vaccine) is recommended as the first booster… It is for those who have not yet taken a precautionary dose.”
ALSO READ | Decision on nasal vaccine for children only after Phase 2, 3 trial on adults: Bharat Biotech CMD
One must not get vaccinated with iNCOVACC if:
- You had a severe allergic reaction to any ingredients of the vaccine
- You had a severe allergic reaction after a previous dose of this vaccine
- You currently have an acute infection or fever
What should you mention before taking your vaccination?
One must inform the vaccinator or the supervising officer about all of your medical conditions, including if you:
- Are on regular medication for any illness, for how long and for which condition
- Have any allergies
- Have fever
- Have a coagulation/bleeding disorder or are on a blood thinner
- Are immunocompromised or are on a medicine that affects your immune system
- Are pregnant
- Have received another COVID-19 vaccine
When will COVID-19 nasal vaccine be available and how to opt for it?
iNCOVACC has been already been introduced on CoWin. However, the doses are likely to be rolled out in the fourth week of January 2023.
Once they are available, one can log in on CoWin and select iNCOVACC to be their precautionary dose. Here’s a step-by-step process to do so:
- Visit CoWIN website
- Login using your registered mobile number
- Enter One Time Password (OTP) to verify
- Afer logging in, click on your vaccine status and tap on the available booster dose
- Search for your nearest vaccination centre by entering the pin code or district name
- Select your preferred centre
- Select your preferred date and time to get the nasal vaccine booster dose
- Confirm your slot
What is the cost of one dose of the COVID-19 nasal vaccine?
Bharat Biotech International Limited said its COVID-19 intranasal vaccine iNCOVACC is priced at Rs 800 (excluding 5 percent Goods and Services Tax (GST)) for private markets and Rs 325 (excluding GST) for government supplies.
Provided that private hospitals are permitted to charge up to Rs 150 as an administrative charge for every dose of the COVID-19 vaccine, one dose of this vaccine developed on a technology licensed by the Washington University in St Louis may cost about Rs 1,000 for every recipient, Moneycontrol reported.
What are the benefits of COVID-19 nasal vaccine?
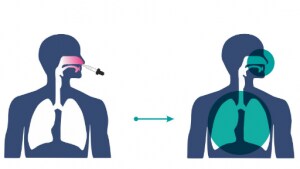
While any injectable vaccine only protects the lower level (of the body), the nasal jab gives protection to the whole body, Krishna Ella, Executive Chairman of Bharat Biotech, had said earlier. It is also designed for efficient distribution and easy pain-free administration, the pharma company said.
As mentioned by Bharat Biotech, the nasal vaccine is:
- Likely to block both infection and transmission of COVID-19
- Non-invasive and needle-free
- Easy to administer as it does not require trained healthcare workers
- Eliminates needle-associated risks (injuries and infections)
- High compliance — ideally suits children and adults
- Scalable manufacturing — able to meet global demand
What are the side effects of the iNCOVACC vaccine?
Side effects that have been reported include:
- Headache
- Fever
- Running nose
- Sneezing
“A severe allergic reaction may very rarely occur after getting a dose of iNCOVACC®. However no such event was reported in the clinical trial with iNCOVACC®,” the Bharat Biotech factsheet says.
What if you have side effects after getting vaccinated with iNCOVACC?
One must contact or visit their health provider/vaccinator/officer supervising their vaccination. They can also immediately go to the nearest hospital.
Moreover, Bharat Biotech says one must report vaccination side effects to the company on toll-free Number: +1 800 102 2245 or email at pvg@bharotbiotech.com .
How successful were trials of iNCOVACC?
Emphasising the success of trials of iNCOVACC vaccine, Krishna Ella had earlier said there is no single instance of side effect or adverse reaction reported so far. Bharat Biotech also claimed that the “intranasal vaccine stimulates a broad immune response”.
“Phase III clinical trial done in 3000 participants, iNCOVACC® has been shown to generate good immunity following two doses given four weeks apart,” the Bharat Biotech factsheet says. “…there is no chance of getting COVID-19 post immunisation with iNCOVACC®,” it adds.
Meanwhile, “there is no scientific information yet available on the appropriateness of use of iNCOVACC® along with other vaccines,” it says. The factsheet also mentions that “individuals with immunosuppression may not generate a full immune response to COVID-19”, adding that “data is not currently available to establish vaccine safety and efficacy in these (immunocompromised) groups.”
iNCOVACC vaccine candidate was evaluated in phase I, II and III clinical trials with successful results, Bharat Biotech, the Hyderabad-based vaccine maker, said in a statement. Clinical trials were conducted to evaluate iNCOVACC as a primary dose schedule as well as a heterologous booster dose for subjects who have previously received two doses of either Covishield or Covaxin.
Phase-III trials of iNCOVACC (as a two-dose regimen) were conducted for safety, immunogenicity in approximately 3100 subjects, in 14 trial sites across India while Heterologous booster dose studies were conducted for safety and immunogenicity in 875 subjects, the release further said.
When did this vaccine get approval from the government?
iNCOVACC is “the world’s first intranasal vaccine” to receive approval for the primary two-dose schedule, and as a heterologous booster dose, Bharat Biotech said in a press release.
It had received approval of the Drugs Controller General of India (DCGI) in November for restricted use in an emergency situation for those above 18 years as a heterologous booster dose.
Earlier this month, Bharat Biotech received approval from the Central Drugs Standard Control Organisation (CDSCO) for the use of heterologous booster doses of iNCOVACC.

Elon Musk forms several ‘X Holdings’ companies to fund potential Twitter buyout
3 Mins Read
Thursday’s filing dispelled some doubts, though Musk still has work to do. He and his advisers will spend the coming days vetting potential investors for the equity portion of his offer, according to people familiar with the matter

KV Prasad Journo follow politics, process in Parliament and US Congress. Former Congressional APSA-Fulbright Fellow









 Listen to the Article
Listen to the Article  Daily Newsletter
Daily Newsletter